Concentrated solar power-driven thermochemical carbon dioxide
and water splitting
Two-step, metal oxide solar thermochemical cycles offer promising pathways to utilize the entire solar spectrum and store it in energy-dense chemical forms, without requiring precious catalysts. At high temperatures and/or low oxygen partial pressures, the metal oxide undergoes partial oxygen depletion or nonstoichiometric reduction. Subsequently, the reduced metal oxide grabs oxygen atoms from carbon dioxide (and/or water) at lower temperatures and/or higher oxygen partial pressures.
The challenge is to optimize metal oxide thermodynamics, and reactor design and operation to maximize the solar-to-fuel conversion efficiencies. We are interested in probing the surface kinetics of oxygen uptake/release for leading metal oxides including perovskites for a wide range of operating temperatures and oxygen partial pressures with various reducing and oxidizing agents. Ultimately, this knowledge is applied in the development of multiphysics computational models that guide optimal reactor design.
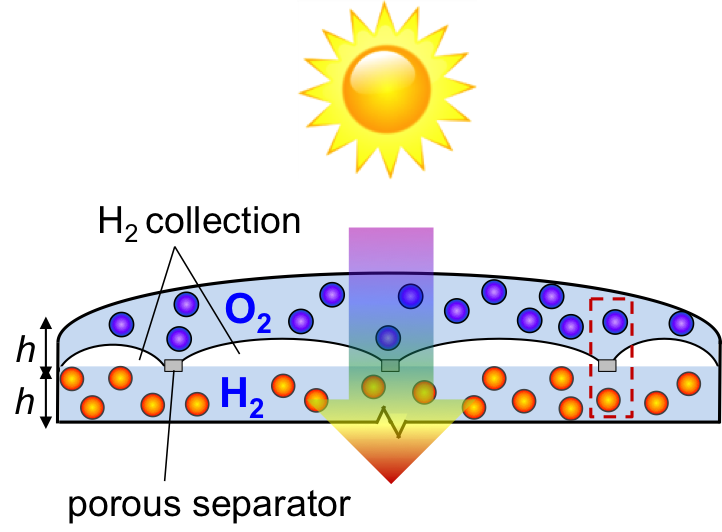
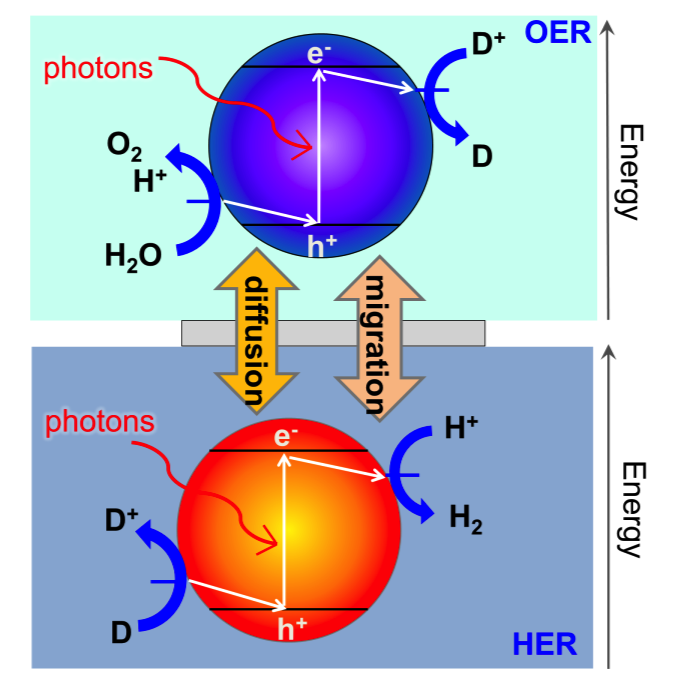
Particle-suspension reactors for photoelectrochemical water splitting
Freely suspended semiconductor powder/particles in water can be tailored to volumetrically absorb incident light, generate charge carriers and drive water electrolysis at the particle-electrolyte interface. Z-scheme water splitting is a concept inspired by natural photosynthesis that divides the thermodynamic penalty to split water between two photo-excitation steps. A reversible redox couple, D+/D, is utilized to relay electrons between the two light absorbers. The challenge in particle-suspension reactor designs is that the back reactions of the redox couple are thermodynamically more favorable than water oxidation and proton reduction, and therefore, kinetic control of the surface reactions is key to achieving efficient hydrogen generation. We are interested in answering the central scientific questions of how the intrinsic semiconductor and cocatalyst material properties, and various surface treatments impact the interfacial charge transfer and reaction kinetics.